Our research group is working on various areas of pure and applied
organic and mechanistic photochemistry


Photooxygenation of organic compounds
We are using different oxygen species such as triplet oxygen ("type I photooxygenations"), singlet oxygen in "type II photooxygenations", and the superoxide radical anion in photo electron transfer initiated (PET) reactions ("type III photooxygenations"). These reactions can be useful for the activation of several substrate classes such as electron-rich or radicalophilic alkenes, dienes or alkynes, enones and a,b-unsaturated carboxylic acid derivatives. By use of non-conjugated, aryl-substituted dienes it is possible to design bicyclization reactions with subsequent oxygen trapping in order to synthesize bicyclic endoperoxides and by a singlet oxygen approach also 1,2,4-trioxanes. This research has found actual applications in the synthesis of potential anti-Malaria compounds which are structurally related to artemisinin (qinghaosu), the antimalarial active component from a plant extract used in China for over 2000 years. These projects are currently funded by a university grant in collaboration with the faculty of medicine.
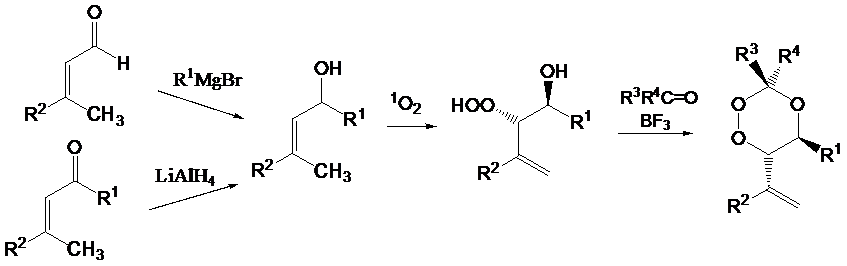
An additional project is the use of new media for performing photooxygenation
reactions: polymer beads and polymer films,
organized media such as microemulsions, zeolites, inverse micelles. These
projects are currently funded by two DFG-projects.
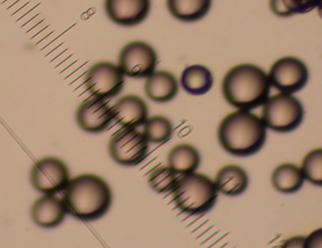


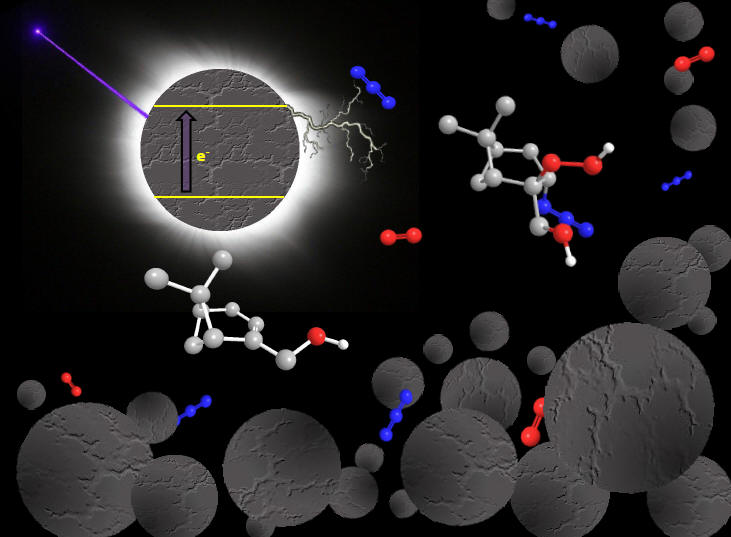
Left:
polymer beads
"microcontainers"
with
a
substrate
and
a
porphyrine dye
(PS-DVP-TPP), average bead diameter 120
mm.
Middle:
TTP-loaded
commericial
polystyrene beads (swollen with EtOAc
in a
petri dish)
irradiated with a halogen lamp.
Right:
synthetic
polymer "nanocubes"
with
a
substrate
and
a
porphyrine dye
(PS-DVP-TTP),
average bead diameter
250 nm.
Recent publications:
"Bicyclic Peroxides and Perorthoester with 1,2,4-Trioxane Structures" Axel G.
Griesbeck, Dirk Blunk, Tamer El-Idreesy, and Angela Raabe, Angew. Chem.
Int. Ed. 2007, 46, 8883-8886.
"Antimalarial Peroxide Dyads from Natural Artemisinin and Hydroxyalkylated 1,2,4-Trioxanes" Axel G. Griesbeck, Jörg Neudörfl, Achim Hörauf, Sabine Specht und Angela Raabe, J. Med. Chem. 2009, 52, 3420-3423.
Photocycloaddition reactions: stereoselectivity and spinselectivity
The factors determining the stereochemical course of triplet photocycloaddition reactions are still a matter of debate. For understanding the configuration of the products resulting from triplet reactions, the knowledge of the geometries where the molecule crosses from the triplet to the S0 surface is essential. It is expected, that there is a structural dependency between selectivity and spin-orbit coupling (SOC) elements which are responsible for the spin inversion process in these tight 1,4-triplet biradical geometries. Our principal objective is to elaborate these structural factors by investigating the effect of substrate variations, temperature effects, radical clocks, and heavy atom effects on the diastereoselectivity of Paternò-Büchi and de Mayo reactions. Especially interesting (and still quite dubious) are cycloaddition reactions with aromatic substrates which in some cases proceed with extremely high regio- and diastereoselectivity. In order to learn more about the contribution of ground state conformations versus SOC geometries we have also started to calculate energies and SOC values by means of ab initio methods. We are also studying the results of magnetic isotope effects on the stereoselectivity of photochemical reactions, a real terra incognita.
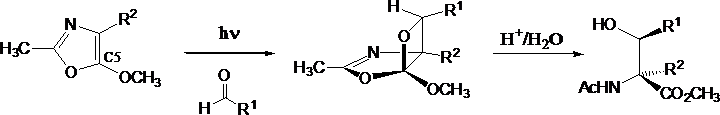
An additional project is the use of these reactions for the synthesis of stereochemically complex organic molecules, such as b-hydroxy a-amino acids or bis-quaternary erythro b-hydroxy aspartate esters using heteroaromatic substrates as oxazoles, thiazoles or isoxazoles.
Recent publications:
"Selectivity control by weak forces: regio- and
stereochemistry of triplet photocycloadditions as a powerful tool for mapping
ISC phenomena,
Axel G. Griesbeck,
Manabu Abe and Samir Bondock, Accounts of Chemical Research 2004,
37, 919-928.
Photodecarboxylation, photoisomerization, and photocyclization reactions of imides
Phthalimides and related molecules are highly interesting substrates for (a) the protection of functional groups, (b) material science (polyimide structures), and (c) photochemistry. Combination of (a) and (c) leads to a useful synthetic approach for modification of substrates from the "chiral pool". Especially alpha-amino acids have been intensively investigated in the last years in order to a) transform these compounds into new derivatives, b) synthesize unnatural amino acids, and c) study asymmetric induction in photochemical reactions. One useful example is the transformation of alkyl-substituted amino acids into the corresponding b,g-unsaturated derivatives. Another approach is the use of the photodecarboxylation of omega-amino carboxylic acids leading to CC-bond forming steps. These processes can be applied for the synthesis of benzopyrrolizidines, pyrrolizidines and indolizidines. These projects are currently funded by a binational DFG-CNRS-grant.
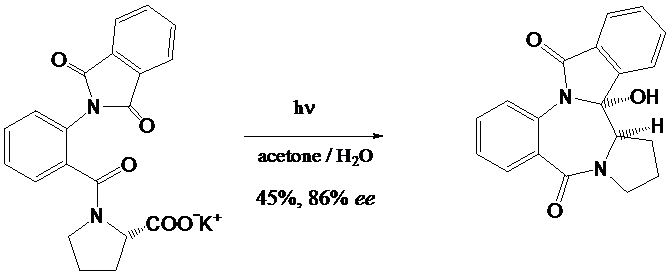
Recent publications:
PET-induced decarboxylative benzylation of
N-alkylphthalimides: A concise route to the aristolactam skeleton,
Axel G. Griesbeck,
Klaus-Dieter Warzecha, Jörg M. Neudörfl und Helmut Görner, Synlett
2004,
2347-2350.
"Novel
2,4-Methanoadamantane-Benzazepine by Domino Photochemistry of N-(1-adamantyl)phthalimide"
Nikola Basarić,
Margareta Horvat, Kata Mlinarić-Majerski,
Elmar Zimmermann, Jörg
Neudörfl, and
Axel G. Griesbeck,
Organic Letters
2008, 10, 3965-3968.
Photoinduced Electron Transfer (PET) and photochemical macrocyclization reactions
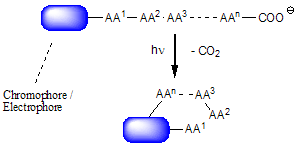
In the course of our investigations of phthalimide photochemistry we discovered a surprisingly efficient activation of omega-amino carboxylic acids, i.e. the macrocyclization of these compounds by way of PET decarboxylation and biradical combination. High dilution conditions which normally are the essential prerequisite for these C-C bond forming reactions are not necessary here. We propose ground-state chelation effects to be responsible for this phenomenon and currently try to strengthen this assumption by NMR, IR, UV and X-ray crystallographic methods. Recent applications are the syntheses of benzodiazepines, benzoazepinediones (medium ring sizes) as well as the synthesis of cyclopeptides and cyclic amines and lactames with ring sizes up to 36.
 |
  |
Recent publications:
"Photoinduced electron transfer chemistry: from studies on PET processes
to applications in natural product synthesis" Axel G. Griesbeck, Norbert Hoffmann,
and
Klaus-Dieter Warzecha, Acc. Chem. Res. 2007, 40, 128-140.
"Photoinduced Azidohydroperoxidation of Myrtenyl Hydroperoxide with
Semiconductor and Lucigenin as PET-Catalysts"
Axel G. Griesbeck, Melissa Reckenthäler und
Johannes Uhlig,
Photochem. Photobiol. Sci.
2010, 9, DOI:
10.1039/c0pp00033g.
New photochemical reactors
and technologies
The application of photochemistry in organic synthesis has several drawbacks. One very serious problem is the scale-up of these reactions and the techniques available to perform photochemical reactions in the 100 mmol or > 1 mol scale. One promising new technique which is based on the excimer emission effect and the first applications of this technique in surface drying and water detoxification, is the use of the "cold" emission lamps produced mainly for photolithographic applications.
Additionally to these activities, our group is investigating photo-electron transfer initiated as well as biradical reactions of substrates from the pool of enantiomerically pure natural compounds ("chiral pool") as well as the photochemistry of carbonyl compounds, alkenes and arenes.Our instrumentation can be used for photochemical reactions on analytical (for quantum yield and efficiency determination by merry-go-round photolyses), semipreparative, and preparative (10-50 g) scale using a newly designed 3 kW XeCl excimer radiation system.
Recent publications:
The excimer radiation system: a powerful tool for
preparative organic photochemistry. A technical note,
Axel G. Griesbeck, Samir Bondock, Nesmine Maptue and
Michael Oelgemöller Photochem. Photobiol. Sci.,
2003, 2, 450-451.
"Das Excimer-Belichtungssystem: Ein nützliches
Werkzeug für die präparative organische Photochemie"
Axel G. Griesbeck,
ChemCologne 2008, 2, 10-11.